REACH in Brief
- REACH is a new regulation that entered into force across Europe on June 1 2007, replacing several other EU chemical laws.
- Importantly, "no data no market”. As an EU-based manufacturer or importer, if you need to register but fail to do so then your must suspend your EU business until Registration is completed.
- REACH is substance based and compliance is the responsibility of each EU legal entity involved in the manufacture or import of that substance.
- All EU manufacturers and EU importers of a substance at ≥1 mt per registrant per year will need to submit a comprehensive registration to the European Chemicals Agency (ECHA) based in Helsinki.
- For existing substances, pre-registration will allow a temporary exemption period of up to mid 2018, the timing of which depends on the registrant's annual tonnage of the substance.
- Authorisation and, ultimately, restriction of substances of high concern for specific uses, where the risks can be demonstrated to be controlled.
- Ongoing review and update of individual registrations as tonnage, identified uses or risk profiles change
Timetable and Milestones
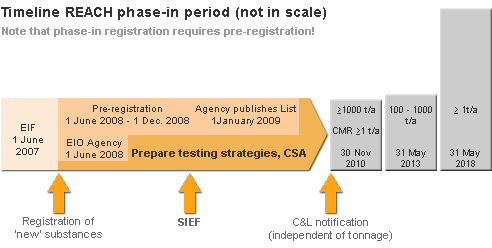
- June 1 2007 - REACH entered into force
- June 1 2008 - start of pre-registration
- December 1 2008 - end of pre-registration
- Early 2009 - publication of SIEF membership
- December 1 2010 - registration deadline for ≥1000 tpa
registration deadline for CMR ≥1 tpa and PBT/vPvB (R50:53) ≥100 tpa
- June 1 2013 - registration deadline for ≥100tpa
- June 1 2018 - registration deadline for ≥1tpa

